Semaglutide Rybelsus, Ozempic, NN9535, OG217SC, NNC 0113-0217 Kutsvaga kushandiswa chete
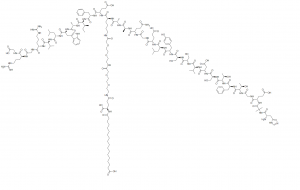
Semaglutide, inotengeswa pasi pemazita emhandoOzempic,WegovyuyeRybelsus,ndi aantidiabetic mushongainoshandiswa pakurapa kweType 2 chirwere cheshugauye seanti-obesity mishongakwenguva refuuremu kutarisira, yakagadzirwa naNovo Nordiskmuna 2012. Semaglutide is aGLP-1 receptor agonist, zvichireva kuti inotevedzera zvinoitwa nemunhuincretin glucagon-yakafanana peptide-1(GLP-1), zvichibva zvawedzerainsulinkuwedzera uye kuwedzerashuga yeropakuraswa nekuvandudzaglycemic control.Zvikonzero zvinosanganisira kuda kurutsa, kurutsa, manyoka, kurwadziwa nemudumbu, uye kupererwa. Muna Zvita 2017, iyo injectable version inonzi Ozempic yakabvumidzwa.MunaGunyana 2019, vhezheni inogona kutorwa nemuromo (Rybelsus) yakabvumidzwa, uye muna Chikumi 2021, jekiseni repamusoro-dose rakatengeswa pasi pezita rekuti Wegovy rekutonga huremu kwenguva refu muvakuru rakabvumidzwa neUS.Food and Drug Administration(FDA).Muna Ndira 2023, FDA yakapa Novo Nordisk mvumo yekudzokorora chinyorwa kuratidza kuti Rybelsus yemuromo inogona kushandiswa senge.kurapa kwekutangakune vanhu vakuru vane chirwere cheshuga chechipiri-zvinoreva kune vanhu vasina kumbotora imwe mishonga yechirwere cheshuga. Muna 2020, semaglutide yaive 129th yaiwanzo kunyorerwa mushonga muUnited States, iine anopfuura mamirioni mana emishonga. Mashoko anoreva zvakafanana: Rybelsus, Ozempic, NN9535, OG217SC, Yekushandisa tsvakurudzo chete.
Biological Activity
| Tsanangudzo | Semaglutide (Rybelsus, Ozempic, NN9535, OG217SC, NNC 0113-0217), glucagon-senge peptide 1 (GLP-1) analogue, inobata kwenguva refu.GLP-1 receptoragonist ine mukana wekurapa kwerudzi rwechipiri chirwere cheshuga mellitus (T2DM). |
| Zvinangwa | GLP-1 receptor |
| In vitro | Semaglutide inosarudzwa seyakanakisa kamwechete pavhiki mumiriri.Semaglutide ine maviri amino acid inotsiva inofananidzwa neyemunhu GLP-1 (Aib8, Arg34) uye inotorwa pa lysine 26. Iyo GLP-1R hukama hwemaglutide (0.38 ± 0.06 nM) yakadzikira katatu kana ichienzaniswa ne liraglutide, nepo albumin affinity. yakawedzera. |
| In vivo | Iyo plasma hafu yehupenyu ndeye 46.1 h mu mini-nguruve inotevera iv kutungamirirwa, uye semaglutide ine MRT ye63.6 h mushure me sc dosing kune mini-nguruve. |
Protocol (kubva pachirevo)
| Tsvagiridzo yeSero: | ● Mitsara yesero:BHK masero ● Pfungwa:0.01 pM - 0.1 μM ● Incubation Nguva:3 h ● Nzira:Frozen aliquots emasero eBHK anoratidza ese hGLP-1R uye CRE firefly luciferase (clone FCW467-12A/KZ10-1) anonyungudutswa, anogezwa kaviri muPBS, uye akamiswa muassay buffer.Masero akaputirwa mu96-tsime mahwendefa pa5000 masero / zvakanaka muhuwandu hwe50 μL.Macompounds ekuedzwa anonatswa mu assay buffer uye 50 μL aliquot inotamirwa mundiro ine maseru kuti isvike yekupedzisira assay concentration ye1 × 10.−14− 1 × 10−7M. Indiro inoputirwa kwe3 h pa5% CO2pa 37 °C.Iyo ndiro yakatenderwa kumira pakamuri tembiricha kwemaminetsi gumi nemashanu isati yawedzera 100 μL ye steadylite plus reagent.Iyo ndiro yakavharwa kuti idzivirire kubva pachiedza uye inozununguswa pakupisa kwekamuri ye30 min.Iyo ndiro inoverengwa muTopCount NXT chiridzwa. |
Solubility (25°C)
| In vitroBatch: | DMSO | 3 mg/mL(0.73 mM) |
| Ethanol | Insoluble | |
| Mvura | Insoluble |
Chemical Information
| Molecular Weight | 4113.58 | ||
| Formula | C187H291N45O59 | ||
| CAS Nha. | 910463-68-2 | ||
| Storage | 3 years | -20°C | upfu |
| 2 years | -80°C | mu solvent | |
| Shipping | Room tembiricha kutumira(Hunhu husina hanya: chigadzirwa chakanaka pa37 ℃ kweinenge vhiki 1.) | ||
Clinical Trial Information
| Nhamba yeNCT | Kupinza vanhu basa | Kupindira | Conditions | Sponsor/Vashandi | Zuva Rokutanga | Zvikamu |
| NCT05537233 | Haisati yatora basa | Mushonga: Semaglutide|Mushonga: Placebo | Type 1 Diabetes|Kufutisa | Yunivhesiti yeColorado Denver | Juvenile Diabetes Research Foundation | Ndira 1 2023 | Phase 2 |
| NCT04885634 | Haisati yatora basa | Zvinodhaka: Semaglutide Injectable Product | Mushonga: Placebo | Atrial Fibrillation | Kuwandisa uye Kufutisa | Axel Brandes|Herlev neGentofte Hospital|Hillerod Hospital Denmark|Svendborg Hospital|Chipatara cheSouth West Jutland|Odense University Hospital | Gumiguru 2022 | Chikamu chechitatu |
| NCT05579977 | Kutsvaga | Drug: PF-07081532|Zvimwe: Placebo|Mushonga: Rybelsus | Diabetes Mellitus|Kufutisa | Pfizer | Gumiguru 27 2022 | Phase 2 |
| NCT05254314 | Kutsvaga | Zvinodhaka: Semaglutide Pen Injector 2.4mg vhiki nevhiki | Zvimwe: Placebo | Asthma | Vanderbilt University Medical Center | National Institute of Allergy uye Utachiona Zvirwere (NIAID) | Gunyana 7 2022 | Phase 2 |
| NCT05478252 | Kutsvaga | Zvinodhaka: Semaglutide J | Mushonga: Semaglutide B | Chirwere cheshuga mellitus Type 2 | Novo Nordisk A/S | Nyamavhuvhu 3 2022 | Chikamu chechitatu |
(data kubvahttps://clinicaltrials.gov, yakagadziridzwa muna 2022-11-29)